For the analysis of any thermodynamic problem, the concerned matter or a region is considered as the system. By definition, thermodynamic system is defined as a definite quantity of matter or a region in space upon which the attention is focused for the analysis of a problem. Everything in the entire universe external to this system is called the surroundings. The system is separated from the surroundings by a boundary. A system boundary is may be either physical or imaginary. It can be either fixed or movable. Thus its volume can be fixed or varying.
A thermodynamic system can interact with the surroundings in two different ways – (i) energy interaction, and (ii) mass interaction. Energy interaction can take place in several forms – such as heat transfer, work transfer, electrical energy transfer, etc. However, majority of the thermodynamic analysis in mechanical engineering field is based on heat and work transfer. An open system allows both energy and mass transfer between the system and surroundings. A closed system allows only energy interaction but no mass interaction. An isolated system does not allow any interaction between the system and surroundings. All such interactions between the system and surroundings occur through the boundary.
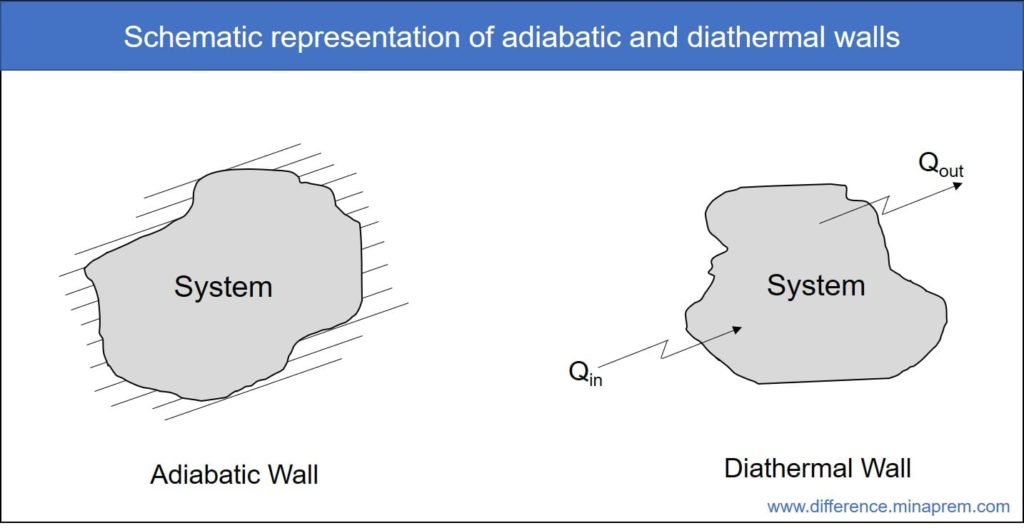
Heat is one form of energy (thermal energy). Sometimes system boundary allows transfer of heat across it, while sometimes it does not allow flow of heat. Based on the feasibility of heat transfer across system boundary, it can be classified into two distinct groups, as enlisted below.
- Adiabatic boundary
- Diathermal boundary
Adiabatic wall or adiabatic boundary
All such boundaries of thermodynamic system that are impermeable to the flow of heat across itself are called adiabatic boundaries. Therefore, a system with adiabatic wall can neither absorb heat from surroundings nor reject heat to the surroundings. However, an adiabatic wall allows energy transfer in other form (such as work transfer).
Examples of adiabatic boundary: There exists no perfect adiabatic wall in this world as heat transfer by radiation cannot be made zero so long as there exists temperature difference between the system and surroundings. However, a boundary made of thermally insulative material with integrated radiation shields can be considered as adiabatic wall for practical purposes.
Diathermal wall or diathermal boundary
All such boundaries of thermodynamic system that permits flow of heat across itself are called diathermal boundaries. Therefore, a system with diathermal wall can absorb heat from surroundings and can also reject heat to the surroundings. As usual, a diathermal wall also allows energy transfer in other form (such as work transfer).
Examples of diathermal boundary: Any physical or imaginary boundary is theoretically diathermal wall. A boundary made of thermally conductive material can be perfect example in this matter if heat transfer by conduction is dominating.