Energy may exist in different forms. It can be light energy, thermal energy, potential energy, kinetic energy, chemical energy, nuclear energy, etc. Every physical matter (or body or thermodynamic system) intrinsically possesses certain amount of energy in one form or another. Such energy can be converted from one form to another for storing it within the same body. It can also be transferred from one body to another with or without changing the form. Every system or body that has absolute temperature above 0 K inherently contains certain amount of thermal energy owing to incessant random motion of its molecules. By definition, “heat” is that part of the thermal energy which can be transferred from one body to another owing to their temperature difference only. Thermal energy from a body can be transferred to other body in two basic ways, either through heat transfer or through work transfer. Out of these two ways, heat transfer spontaneously occurs only because of temperature difference. Accordingly, “temperature” is one property of the thermodynamic system by virtue of which heat can be transferred. Temperature cannot be directly transferred. It is only heat that can be transferred. This heat transfer can, however, alter temperature of a system or body.
Further, total heat content within a body cannot be measured; it can only be measured when it is transferred from one body to another. That means, only the amount of heat gained by a body or discharged from the body can be measured. That is why heat is called a boundary property. On the contrary, actual temperature of any system at a specific condition can be measured. Accordingly, temperature is called a property of the system. Heat transfer between two bodies does not depend on the amount of heat possessed by the bodies; rather it relies on their temperature. Heat transfer always spontaneously takes place from a hotter body (higher temperature) to a colder body (lower temperature) regardless of their heat content. Temperature of a body increases if it only gains heat, and temperature decreases if a body only discharges heat (provided that there is no other form of energy exchange). So temperature change is the result of heat transfer. Coming back to basic science, temperature is a fundamental property standardized in SI or metric system of units. Unit of temperature, Kelvin (K), is one fundamental unit. On the other hand, heat is a derived quantity similar to any other form of energy, and unit of heat (Joule or Calorie) is also a derived unit. Various similarities and differences between heat and temperature are given below in table format.
Similarities between heat and temperature
- Both heat and temperature are scalar quantities. A scalar has only magnitude, while a vector has both magnitude and direction. Although heat is actually a flow property, heat is actually a scalar. The rate of heat flow (called heat flux) is presented by the gradient of temperature (Fourier’s law), and the gradient of any scalar is a vector. So “heat flux” is vector, but heat is a scalar like temperature.
- Both are measureable, although in different ways. They are also quantifiable.
- Usually both are interrelated; however, occurrence of one is also possible without affecting the other. For example, temperature of an object can be varied without transferring heat but by exchanging work (another form of energy).
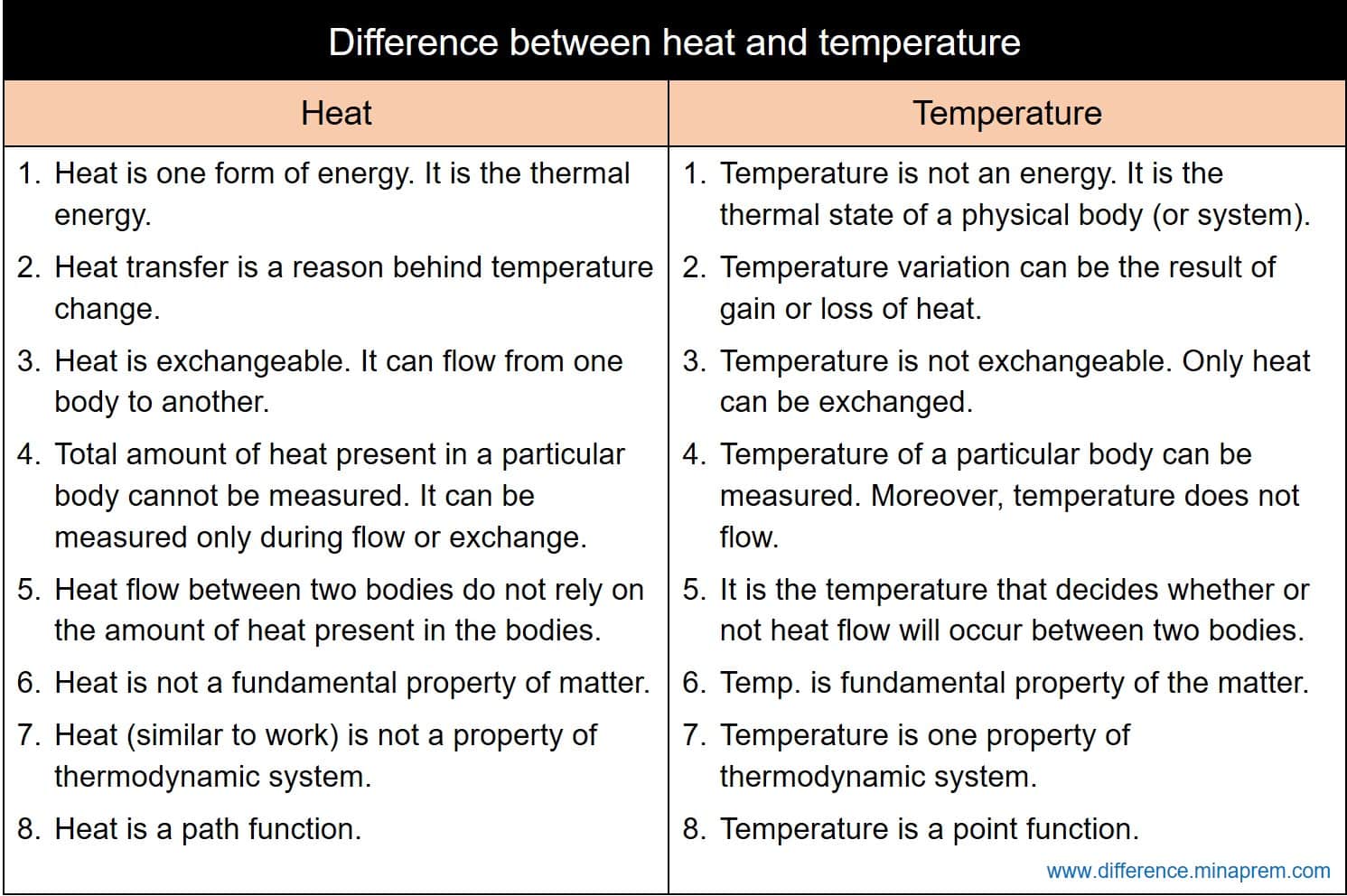
Differences between heat and temperature
| Heat | Temperature |
|---|---|
| Heat is one form of energy. It is the thermal energy. | Temperature is not an energy. It is the thermal state of a physical body (or thermodynamic system). In classical mechanics, temperature of a body indicates the average kinetic energy of all the molecules of the corresponding body. |
| Heat flow is a reason behind temperature change. | Temperature variation can be the result of gain or loss of heat. |
| Two bodies having same temperature may not necessarily contain same quantity of heat (as heat capacities are mass dependent). | Two bodies having same heat may not necessarily have same temperature. |
| Heat is exchangeable. It can flow from one body to another. So a particular body can release or gain certain amount of heat. | Temperature is not exchangeable. Only heat can be exchanged, and the result of heat transfer can be the variation in temperature. |
| Total amount of heat present in a particular body cannot be measured. It can be measured only during flow or exchange. Thus gain or loss of heat (i.e. amount of heat flow between two bodies) can be measured. | Temperature of a particular body can be measured. Moreover, temperature does not flow (it is only heat that can flow). |
| Amount of heat transferred between two bodies can be measured by Calorimeter. | Temperature of a body can be measured by Thermometer. |
| Unit of measurement of heat is: Joule (J) in SI system or Calorie (Cal) in CGS system. | Unit of measurement of temperature is degree centigrade (°C) or Kelvin (K). |
| Its dimension is [M L2 T–2]. | Its dimension is [θ]. |
| Heat is not a fundamental property of matter. It is one derived property, and its unit is also one derived unit. | Temperature is a fundamental property of the matter. Its unit (Kelvin, K) is also a fundamental unit (or base unit). |
| Heat (similar to work) is not a property of thermodynamic system. It is a flow property. Heat capacities and specific heat capacities are, however, properties of thermodynamic system. | Temperature is one property of thermodynamic system. |
| Heat is a path function. So it relies on the path taken by a thermodynamic system to reach one state from another. | Temperature is a point function. So it is independent of the path followed by the system to reach one state from another. Every thermodynamic state has a fixed definite value of temperature. |
| The fact that whether heat will flow from one body to another body is not governed by the amount of heat present in the bodies. | It is the temperature that decides whether or not heat flow will occur between two bodies. Heat always flows from a high temperature body to a low temperature body. |
| Heat capacities (not heat) depend on the mass of the system. So these are an extensive properties. However, specific heat capacities are intensive properties. | Temperature is independent of mass; so it is an intensive property. |