Nuclear fission is one type of nuclear reaction where a heavier nucleus, when bombarded by neutron of sufficient velocity, splits into two or more lighter nuclei and at the same time releases one or more neutrons and energy. The neutrons produced in one fission reaction can once again participate in fission of the other nucleus of fissile materials leading to a self-sustained chain reaction. The cumulative mass of the reaction products is lower than the mass of the reactants. The consequent mass defect ultimately leads to the generation of thermal energy following the Einstein’s Mass-Energy Conversion Formula (E = mc2), and hence (mass + energy) remains conserved. All such chemical elements having mass number higher than iron can theoretically undergo nuclear fission. This reaction is utilized in nuclear fission power plants where uranium fuel (enriched with U-235) is made to undergo fission in a reactor in order to generate heat. Such heat is used to produce high pressure steam for driving a turbine, which, in turn, generates electricity.
Another type of nuclear reaction is nuclear fusion where two or more lighter nuclei are combined to form a heavier nucleus. Although (mass + energy) remains conserved in nuclear fusion, but the cumulative mass of the reaction products is lower than the cumulative mass of reactants, and hence mass defect occurs. As usual, this mass defect is the source of energy released in fusion reaction. The energy release when calculated per unit nucleon (proton and neutron) of the reactant in fusion is significantly higher than that obtained in fission. Thus fusion offers high energy density. Deuterium (Hydrogen-2 isotope) is considered as common fuel for fusion, even though it is also possible with protium (H-1) or tritium (H-3) isotopes. Whereas high velocity neutrons are required to initiate nuclear fission, very high temperature, a critical ion density and sufficient confinement time are required to initiate fusion reaction. Nuclear fusion is the source of extreme energy of the stars including the Sun. To the fullest advancement of science in today’s scenario, it is not possible to control the fusion reaction efficiently after its initiation. Thus it lacks wide application towards mankind (such as in power plants). Various similarities and differences between nuclear fission and nuclear fusion are given below in table format.
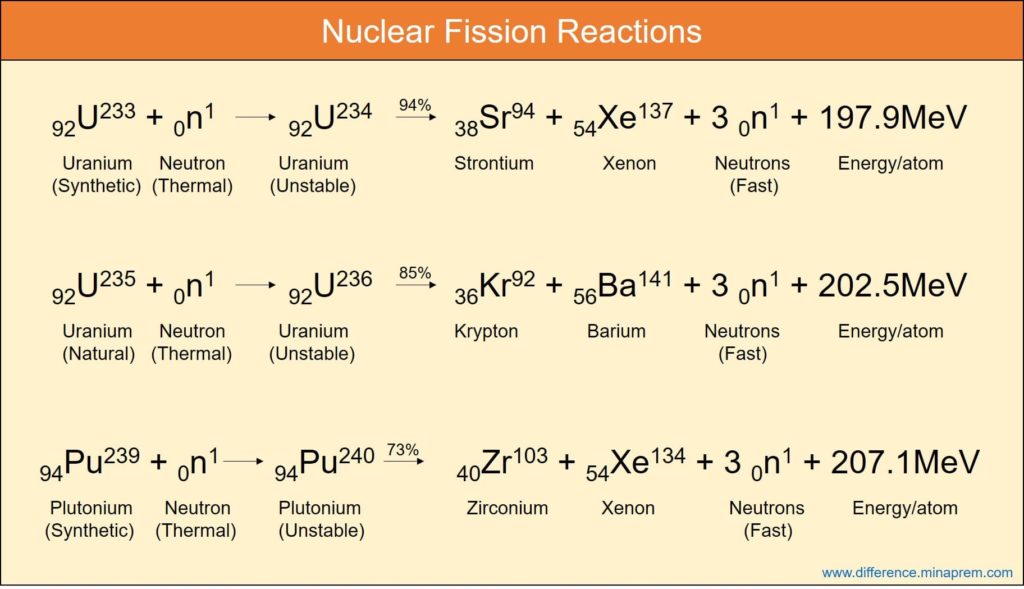 Similarities between nuclear fission and nuclear fusion
Similarities between nuclear fission and nuclear fusion
- Both fission and fusion reactions are nuclear phenomenon and thus nucleus of the atoms participate in reactions.
- New element formation occurs in both the reactions owing to nucleon rearrangement.
- Mass defect takes place in each type of reaction. This mass defect is primary source of heat generation following the Einstein’s formula (E = mc2).
- External environment such as pressure, temperature, density, etc. cannot influence the rate of fission or fusion reaction so long as the necessary conditions are achieved.
- Both the reactions can be employed for mankind, such as for electricity generation in nuclear power plants. In today’s world, fission power plants are common, while fusion plants lack extensive application as such plants are still under development.
Differences between nuclear fission and nuclear fusion
| Nuclear Fission | Nuclear Fusion |
|---|---|
| In nuclear fission reaction, a heavier nucleus splits into two or more lighter nuclei due to the bombardment of neutron. | In nuclear fusion reaction, two or more lighter nuclei get combined to produce a heavier nucleus. |
| Nuclear fission was first discovered by scientists Otto Hahn and Fritz Strassmann. | Nuclear fusion was first discovered by scientist Hans Bethe. |
| Energy release per atom in fission is comparatively higher. | Energy release per atom of fusion is usually lower. |
| Energy release per unit nucleon is significantly lower. | Energy release per unit nucleon in fusion is higher, and thus fusion offers high energy density. |
| In order to initiate a fission reaction, a high velocity neutron is desired. To sustain the chain reaction, a critical mass of fuel is desired. | Three conditions, namely (i) very high temperature in the order of 107 – 108 K, (ii) critical ion density, and (iii) sufficient confinement time are required to initiate fusion reaction. |
| Nuclear fission is a self-sustained reaction because of its capability to undergo spontaneous chain reaction. | Nuclear fusion is not a chain reaction, so it is not self-sustained (i.e. one fusion incident does not influence another one). |
| Fission is possible for atoms with higher atomic number. Theoretically, iron (Fe) is considered as boundary as it has highest binding energy per nucleon. So all elements in the periodic table after Fe can possibly undergo nuclear fission. | Fusion is possible for atoms with lower atomic numbers. Theoretically, all elements in the periodic table before Fe can possibly undergo nuclear fusion. |
| Fission can be controlled and manipulated effectively, and therefore it can be utilized for several mankind purposes, such as for large scale power generation in nuclear power plant. | To the fullest advancement of science in today’s scenario, it is not possible to control the fusion reaction efficiently after its initiation. Thus it lacks large scale applications towards mankind. |
| Uranium-233, Uranium-235 and Plutonium-239 isotopes are three potential fuel for nuclear reactors that work based on fission. | Deuterium (H-2) and tritium (H-3) are two common isotopes of hydrogen used in fusion reactors (such reactors are still used for trial and research purpose only). |
| Mostly nuclear fission reaction leads to the generation of radioactive materials. | Fusion reaction usually does not generate radioactive materials. This is the biggest benefit of nuclear fusion. |
| Apart from nuclear reactors in power plant, fission reaction is also utilized in several nuclear weapons (notably atom bomb). | Nuclear fusion occurs in the Sun and other stars (in fact, fusion is the source of energy and light of the stars). Uncontrolled fusion is also utilized in hydrogen bomb. |
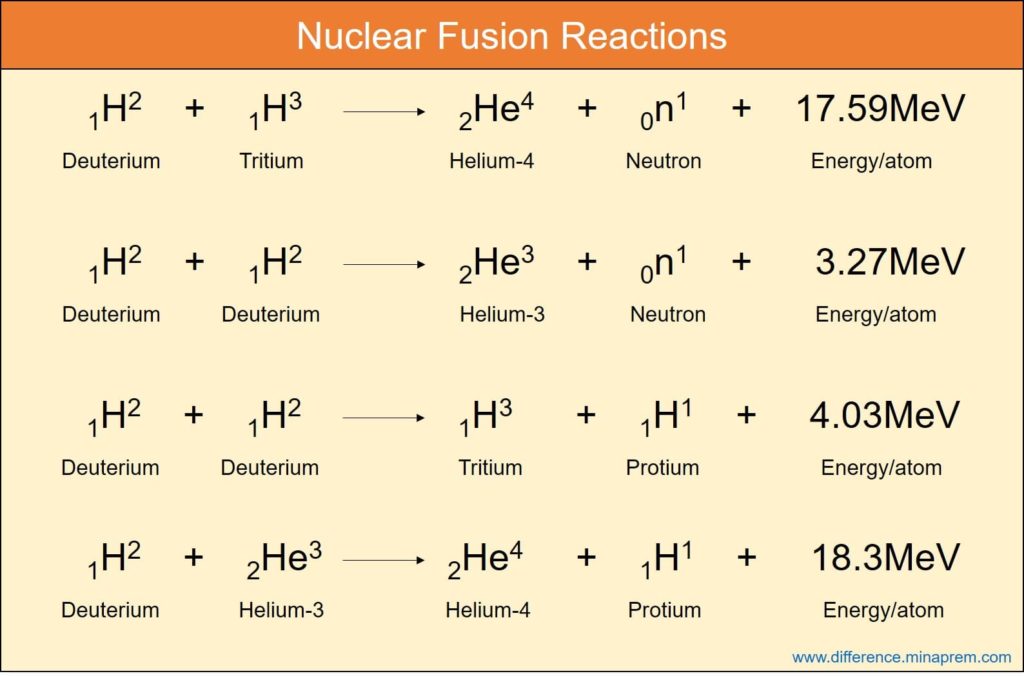
References
- Nuclear Fusion and Fission by F. Y. Brown (2017, Cavendish Square Publishing).
- Nuclear Fission and Atomic Energy by W. E. Stephens (2018, Creative Media Partners).